Redox flow batteries (RFBs) represent a viable solution for large-scale energy storage applications that are capable of storing large amounts of electrical energy from intermittent renewable sources by electrochemical reactions of redox species dissolved in liquid electrolytes at the active surface sites of electrodes. Despite years of experimental efforts, atomic-scale details about the mechanisms of reactions occurring in the electrolyte solutions and at the electrode surfaces in RFBs, required for further enhancement of energy and power density, are still lacking. We are undertaking systematic first-principles studies to understand both solution and interfacial electrochemistry of RFBs. Our current focus is on the chemistry of vanadium RFBs (V2+/V3+ and VO2+/VO2+ redox couples) and electrocatalytic properties of carbon-based (graphite, graphene) electrodes for which a wealth of experimental data is available.
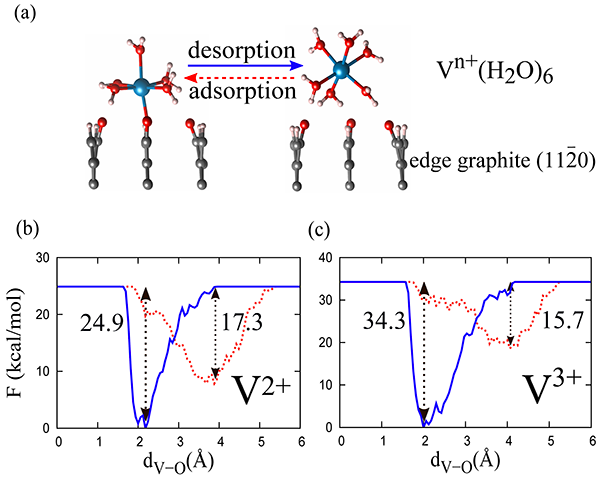
The figure shows free-energy profiles for the adsorption-desorption processes between outer- and inner-sphere complexes of aqueous V2+ and V3+ at the edge graphite as simulated using AIMD-based metadynamics.
Representative Publications
Jiang Z., Klyukin K. and Alexandrov V. “First-Principles Study of Adsorption-Desorption Kinetics of Aqueous V2+/V3+ Redox Species on Graphite in a Vanadium Redox Flow Battery.”
Physical Chemistry Chemical Physics (Communication) 19, 14897-14901 (2017)
Jiang Z., Klyukin K. and Alexandrov V. “Structure, Hydrolysis and Diffusion of Aqueous Vanadium Ions from Car-Parrinello Molecular Dynamics.”
Journal of Chemical Physics 145, 114303-114311 (2016)